
Diane Hawley
Education
B.A., University of Kansas, 1976. Ph.D., Harvard University, 1982 (William R. McClure). Postdoctoral: Rockefeller University, 1983-86 (Robert G. Roeder). Honors and Awards: NSF Predoctoral Fellow, 1976–79; NIH Postdoctoral Fellow, 1983–86; NSF Presidential Young Investigator Award, 1987–92; Searle Scholar Award, 1987–90. At Oregon since 1986.
Research
Hawley’s research group is interested in the enzymology of RNA polymerases and the mechanisms by which eukaryotic transcription is regulated. The goal of ongoing research is to characterize the functional properties of proteins that determine elongation behavior and transcription initiation frequency of mammalian and yeast RNA polymerase II. Recent experiments have focused on three areas: (1) studies of the mechanism of termination and site-specific arrest of transcription elongation and characterization of the intrinsic nuclease activity of RNA polymerase II; (2) investigation of the role of RNA polymerase II in transcription-coupled repair; and (3) protein-protein and protein-DNA interactions that contribute to early stages of preinitiation complex assembly at promoters.
During transcription elongation, RNA polymerase II becomes arrested at specific DNA sites in vitro. Arrested polymerases can be induced to resume transcription by elongation factor SII. Study of the mechanism by which SII relieves arrest led to the discovery of a nucleolytic activity of the RNA polymerase/SII complex. The relationship between this activity and the mechanism of transcriptional arrest and readthrough is being investigated by both biochemical and genetic methods. In addition, other possible functional roles for the nuclease activity (e.g., proofreading) are being explored. We have used a genetic screen in the yeast Saccharomyces cerevisiae to identify RNA polymerase II mutants with reduced nuclease activity. We have also identified RNA polymerase variants that are defective for termination or have reduced accuracy. We are using these altered polymerases to investigate how RNA polymerase II coordinates and regulates its various enzymatic activities.
Chromosomal DNA is under constant attack from environmental agents and endogenous reactive chemical species. The capacity to recognize and repair the resulting DNA damage is essential for cell survival. The consequences of unrepaired DNA damage in somatic cells include cell death, tissue degeneration, aging, and oncogenesis. Unrepaired damage in germline cells can result in deleterious mutations. Transcription-coupled repair (TCR) is an important DNA repair pathway that targets bulky DNA adducts, such as the cyclobutane pyrimidine dimers caused by UV irradiation. These lesions are repaired first in the template strands of actively transcribed genes. The asymmetry of this repair pattern is due to the preferential targeting of repair enzymes by RNA polymerases stalled at DNA lesions. We have found mutant versions of yeast RNA polymerase II that reduce the effectiveness of TCR and we are using these mutants to investigate the role of the RNA polymerase in this important repair pathway.
A number of cellular proteins assemble with RNA polymerase II at promoters. One of these, the TATA-binding protein (TBP), is an unusual DNA-binding protein in that it binds within the minor groove of the DNA helix. TBP also bends the DNA upon binding. Analysis of the binding of TBP to a number of different sequences has shown that the affinity of the TBP-DNA interaction and the apparent bend angle of the complex are directly correlated with transcriptional activity. We are using a variety of biochemical and biophysical methods to characterize the TBP-DNA interaction and the association of the TBP-DNA complexes with other transcription factors.
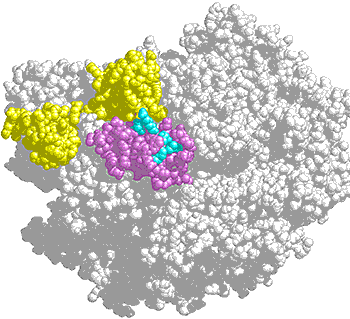
A cluster of amino acids (bright blue) form a suspected protein interaction surface in an external domain (purple) of yeast RNA polymerase II. Mutations in this domain alter termination efficiency. Yellow atoms indicate Rpb9, a subunit important for transcription fidelity.
Publications
Starr, D. B., B. C. Hoopes, and D. K. Hawley. DNA bending is an important component of site-specific recognition by the TATA binding protein. J. Mol. Biol. 250, 434-446 (1995).
Reeder, T. C. and D. K. Hawley. Promoter proximal sequences modulate RNA polymerase II elongation by a novel mechanism. Cell 87, 767-777 (1996).
Foulds, C. E. and D. K. Hawley. Analysis of the human TATA binding protein promoter and identification of an Ets site critical for activity. Nucleic Acid Res. 12, 2485-2494 (1997).
Hoopes, B. C., J. F. LeBlanc, and D. K. Hawley. Contributions of the TATA box sequence to rate-limiting steps in transcription initiation by RNA polymerase II. J. Mol. Biol. 277, 1015-1031 (1998).
Thomas, M., A. A. Platas, and D. K. Hawley. Transcriptional fidelity and proofreading by RNA polymerase II. Cell 93, 627-637 (1998).
