John Keana
Education
B.A., Kalamazoo College, 1961. Ph.D., Stanford University, 1965 (W. S. Johnson). Postdoctoral: Columbia University, 1965 (R. Breslow). Honors and Awards: Phi Beta Kappa; NSF Predoctoral and Postdoctoral Fellow; Eastman Kodak Award; Alfred P. Sloan Foundation Fellow; John S. Guggenheim Foundation Fellow; NIH Research Career Development Award; Member National Cancer Institute Research Manpower Study Section; Consultant, MRI; Consultant, Cambridge NeuroScience, American Cancer Society Panel; Fogerty International Research Review Panel; Consultant, Mallinckrodt Medical; Editorial Board, Bioconjugate Chemistry; Co-Founder, Acea Pharmaceuticals, 1992; Consultant, Abbott Laboratories; Co-Founder, Advanced MicroBotics (Ikonos), 1994; Consultant, CoCensys; Executive Director of Chemistry, CoCensys, 1997; Consultant, Cytovia (now a subsidiary of Maxim Pharmaceuticals), 1999; Co-Founder Axos Pharmaceuticals, 2001; Consultant, Applied Biosystems; Consultant, Patent Litigation, 2002; Consultant, Orexigen, Inc., 2003; Editorial Borad, Drug Design Reviews - Online, 2003; Consultant, Novacea, Inc., 2004-present; Consultant, Ascenta Pharmaceuticals, Inc., 2004-present; Editorial Board, Medicinal Chemistry, 2004-present; Consultant, Novalar, Inc.,2004; Member, Scientific Advisory Board, Metagenics/MetaProteomics, Inc., 2005-present.. At Oregon since 1965.
Statement
Professor Keana has retired from research and teaching activities but continues to serve as a medicinal chemistry/organic chemistry consultant for several small biopharmaceutical companies and several patent law firms.
Research
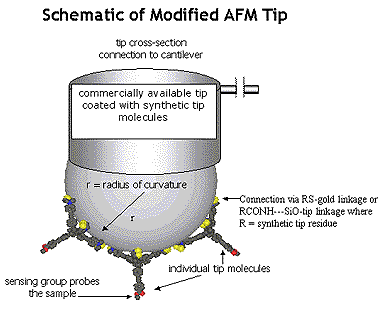
Principal Research Interests: The central theme of John Keana's research program is the design, synthesis, characterization, and collaborative application of novel molecules. Target molecules are chosen for their relevance to the solution of important problems in biochemistry, molecular biology, neuroscience, or medicine and may be either organic, organometallic, or essentially inorganic in nature. The research program is markedly strengthened by frequent collaboration with research groups in the Institute of Molecular Biology and the Department of Physics at the University of Oregon and research groups at Parke-Davis, CoCensys, Inc. (now Purdue Pharmaceuticals), Cytovia, Inc. (a subsidiary of Maxim Pharmaceuticals) and SUNY Brooklyn. The projects below illustrate synthetic objectives typical of this laboratory. Project 1. Atomic force microscopy (AFM) is a relatively recent and rapidly expanding atomic level analytical technique that directly images three-dimensional surfaces of non-conducting substrates. AFM images are formed by reconstructing the contour of force interactions exerted between the scanning tip and the surface. Although not precisely defined, the resolution of AFM is limited by the large tip size relative to the dimensional parameters of individual substrate molecules. Our AFM project involves the design, synthesis, and collaborative application of tower-shaped molecules that have a broad base and that taper to a single atom or functional group. We envisage attachment of these molecules to a conventional AFM tip by way of three-point attachment at the base. Molecular dimensions are such that only one molecule is expected to be oriented suitably for imaging the sample by the tapered end of the tower (see schematic diagram below).
| 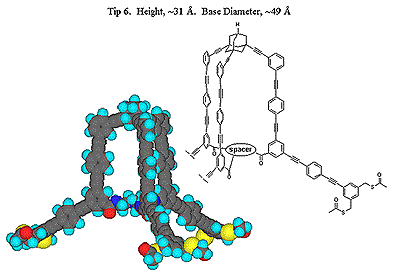 | 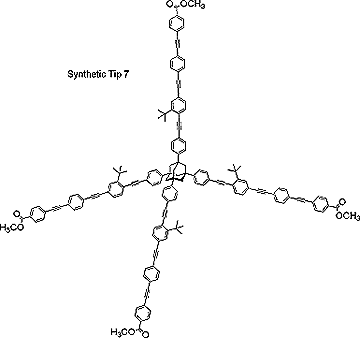 |
Project 2. The phosphatidylinositol-specific phospholipase C (PI PLC) enzymes occupy a central role in cellular function, for example, the release of proteins anchored to the cell surface or amplification of cellular signals such as the binding of a hormone molecule on the cell surface. Progress in the study of the bacterial and the mammalian enzymes has been hampered by the lack of convenient, sensitive means of measuring enzymatic activity. An objective in this laboratory is the design and synthesis of novel substrates and inhibitors for these enzymes. Biochemical and biophysical studies using these new molecules are being done in collaboration with Professor O. Hayes Griffith.

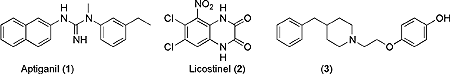
Project 3. A major focus has been the discovery of neuroprotective drugs and the characterization of their receptors. Neuroprotective agents can reduce stroke and other ischemic brain damage when administered hours after the event. Drugs resulting from collaborative research include Aptiganel (1) (phase III trials) and Licostinel (2) (phase I trials). Recent emphasis has been on the discovery of subtype selective NMDA receptor antagonists, for example, piperidine 3. These drugs show promise as agents for the treatment of Parkinson's disease. NMDA receptors may also be involved in pain perception in the spinal cord. This medicinal chemistry project is now essentially complete except for the preparation and submission of several manuscripts. Recent emphasis has been
on the design and synthesis of tumor selective anticancer drugs with a novel
mechanism of activation.
Publications
200. Zaikova, T. O.; Rukavishnikov, A. V.; Birrell, G. B.; Griffith, O. H.; Keana, J. F. W. "Synthesis of Fluorogenic Substrates for Continuous Assay of Phosphatidylinositol-Specific Phospholipase C," Bioconjugate Chem., 2001, 12, 307-313.
201. Zhou, Z.-L.; Navratil, J. M.; Cai, S. X.; Whittemore, E. R.; Espitia, S. A.; Hawkinson, J. E.; Tran, M.; Woodward, R. M.; Weber, E.; Keana, J. F. W. "Synthesis and SAR of 5-, 6-, 7- and 8-Aza Analogs of 3-Aryl-4-hydroxyquinolin-2(1H)-one as NMDA/Glycine Site Antagonists, " Bioorg. Med. Chem, 2001, 9, 2061-2071.
205. Li, Q.; Rukavishnikov, A. V.; Petukhov, P. A.; Zaikova, T. O.; Keana, J. F. W. �Nanoscale 1,3,5,7-Tetrasubstituted Adamantanes and p-Substitutied Tetraphenyl-methanes for AFM Applications,� Organic Lett. 2002, 21, 3631-3634.
206: Zhou, Z. L.; Kher, S. M.; Cai, S. X.; Whittemore, E. R.; Espitia, S. A.; Hawkinson, J. E.; Tran, M.; Woodward, R. M.; Weber, E.; Keana, J. F. W. "Synthesis and SAR of Novel Di- and Trisubstituted 1,4-Dihydroquinoxaline-2,3-diones Related to Licostinel (Acea 1021) as NMDA/Glycine Site Antagonists," Bioorg. Med. Chem. 2003, 11, 1769-1780.
207: Li, Q.; Rukavishnikov, A. V.; Petukhov, P. A.; Zaikova, T. O.; Jin, C.; Keana, J. F. W. "Nanoscale Tripodal 1,3,5,7-Tetrasubstituted Adamantanes for AFM Applications," J. Org. Chem. 2003, 68, 4862-4869.
208. Li, Q.; Jin, C.; Petukhov, P. A.; Rukavishnikov, A. V.; Zaikova, T. O.; Phadke, A.; LaMunyon, D. H.; Lee, M. D.; Keana, J. F. W. "Synthesis of Well-Defined Tower-Shaped 1,3,5-Trisubstituted Adamantanes Incorporating a Macrocyclic Trilactam Ring System," J. Org. Chem. 2004, 69, 1010-1019.
209. Kransnoslobodtsev, A. V.; Shlyakhtenko, L. S.; Ukraintsev, E.; Zaikova, T. O.; Keana, J. F. W.; Lyubchenko, Y. L. "Nanomedicine and protein misfolding diseases," Nanomedicine 2005, 1, 300-305.