
Kenneth Prehoda
Education
B.A., California State University - Sacramento, 1991. Ph.D. University of Wisconsin - Madison, 1997. Postdoctoral: University of California, San Francisco, 1997-2001. Honors and Awards: NIH Postdoctoral Fellow, 2000-2001; Damon Runyon Scholar, 2003-2006. At Oregon since 2001.
Research
Research in the Prehoda lab focuses on the biochemical processes that allow cells to respond to changes in their environment. Environmental cues, or “signals”, pass across a cell’s plasma membrane and initiate a molecular program that associates a signal with an appropriate response. For example, during development stem cells respond to signals from neighboring cells by undergoing a series of precise divisions that lead to the multitude of tissues and organs in complex organisms. Our research attempts to uncover the molecular programs that control highly regulated events like these, and elucidate the mechanisms of information transfer that comprise them. In many instances, protein-protein interactions underlie the regulation of complex cellular processes. Because protein-protein interactions are critically important for cellular signaling, many signaling proteins contain specialized domains that are responsible for binding target proteins.
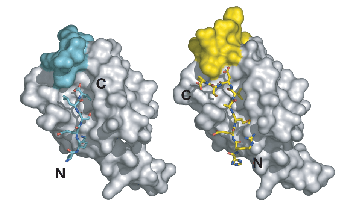
Figure caption: Structures of PDZ domain complexes involved in stem cell division. On the left, the PDZ from the protein Par-6 is bound to a carboxy-terminal sequence (N and C-terminii of the ligand are labeled). This mode of binding is enforced by a steric mechanism – residues that would extend past the c-terminus would clash with residues from the domain itself. On the right, an internal sequence is shown bound to the Par-6 PDZ domain. This sequence bypasses the carboxy-terminal requirement by taking advantage of plasticity in the PDZ domain.
In recent work, we have shown how PDZ protein interaction domains, one of the most common in the human genome, are regulated, and how they specifically bind to carboxy-terminal sequences. We are also studying how interactions between domains in the same protein lead to the complex signaling observed in cells. For example, in the membrane-associated guanylate kinase (MAGUK) family of proteins an intramolecular interaction between an SH3 and GK domain regulates the ability of each domain to bind ligands.
Part of our research program involves determining structures of protein complexes using X-ray crystallography or nuclear magnetic resonance. This structural information is complemented by biochemical, biophysical, and cell biological experiments in order to obtain a complete description of the system under study.
Publications
Prehoda KE. (2009) "Polarization of Drosophila Neuroblasts During Asymmetric Division" Cold Spring Harbor Perspectives in Biology
Marcette J, Hood IV, Johnston CA, Doe CQ, Prehoda KE. (2009) "Allosteric Control of Regulated Scaffolding in Membrane Associated Guanylate Kinases" Biochemistry 48:10014-9.
Johnston CA, Hirono K, Prehoda KE, Doe CQ. (2009) "Identification of an Aurora-A/PinsLINKER/ Dlg Spindle Orientation Pathway using Induced Cell Polarity in S2 Cells" Cell 138:1150-63.
Atwood SX, Prehoda KE. (2009) "aPKC Phosphorylates Miranda to Polarize Fate Determinants during Neuroblast Asymmetric Cell Division" Curr Biol 19:723-9.
Newman RA, Prehoda KE. (2009) "Intramolecular interactions between the SRC homology 3 guanylate kinase domains of discs large regulate its function in asymmetric cell division" J Biol Chem 284:12924-32.
Liu SL, Fewkes N, Ricketson D, Penkert RR, Prehoda KE. (2008) "Filament-dependent and -independent localization modes of Drosophila non-muscle myosin II" J Biol Chem 283:380-7.
Nipper RW, Siller KH, Smith NR, Doe CQ, Prehoda KE. (2007) "Gαi generates multiple Pins activation states to link cortical polarity and spindle orientation in Drosophila neuroblasts" Proc Natl Acad Sci U S A. 2007 104:14306-11.
Atwood SX, Chabu C, Penkert RR, Doe CQ, Prehoda KE. (2007) "Cdc42 acts downstream of Bazooka to regulate neuroblast polarity through Par-6-aPKC." J Cell Sci. 2007 120: 3200-3206.
Peterson FC, Deng Q, Zettl M, Prehoda KE, Lim WA, Way M, Volkman BF. (2007) "Multiple WASP-interacting protein recognition motifs are required for a functional interaction with N-WASP." J Biol Chem. 2007 282:8446-53.
Qian Y, Prehoda KE. (2006) "Interdomain interactions in the tumor suppressor discs large regulate binding to the synaptic protein GukHolder." J Biol Chem. 2006 281:35757-63.
Papayannopoulos V, Co C, Prehoda KE, Snapper S, Taunton J, Lim WA. (2005) "A polybasic motif allows N-WASP to act as a sensor of PIP(2) density." Mol Cell. 2005 17:181-91.
Penkert R.R., Divittorio H.M., Prehoda K.E. (2004) "Internal recognition through PDZ domain plasticity in the Par-6-Pals1 complex." Nat Struct Mol Biol 11:1122-7 (2004)
McGee A.W., Nunziato D.A., Maltez J.M., Prehoda K.E., Pitt G.S., Bredt D.S. (2004) "Calcium channel function regulated by the SH3-GK module in beta subunits." Neuron 42:89-99 (2004)
Peterson F.C., R.R. Penkert, B.F. Volkman, and K.E. Prehoda (2004) Cdc42 regulates the Par-6 PDZ domain through an allosteric CRIB-PDZ transition. Mol Cell 13:665-76.
Volkman B.F., K.E. Prehoda, J.A. Scott, F.C. Peterson, and W.A. Lim (2002) Structure of the N-WASP EVH1 domain-WIP complex: insight into the molecular basis of Wiskott-Aldrich Syndrome. Cell 111:565-76.
Prehoda, K.E. and W.A. Lim. (2002). How signaling proteins integrate multiple inputs: a comparison of N-WASP and Cdk2. Curr Opin Cell Biol 14(2):149-54.
McGee, A.W., S.R. Dakoji, O. Olsen, D.S. Bredt, W.A. Lim, and K.E. Prehoda. (2001). Structure of the SH3-guanylate kinase module from PSD-95 suggests a mechanism for regulated assembly of MAGUK scaffolding proteins. Mol Cell. 8(6):1291-301.
