
Darren Johnson
Education
B.S., University of Texas, Austin, 1996. Ph.D., University of California, Berkeley, 2000 (Kenneth N. Raymond). Postdoctoral: The Scripps Research Institute, 2001-03 (Julius Rebek, Jr.). Honors and Awards: Phi Beta Kappa; NIH Postdoctoral Fellow, 2002-03; NSF CAREER Award, 2006; Research Corporation Cottrell Scholar Award, 2006; University of Oregon Fund for Faculty Members Excellence Award, 2012. At Oregon since 2003.
Research
Research in the group explores problems in coordination chemistry and organic synthesis using the relatively new field of supramolecular chemistry as a tool. Research projects include developing specific metal chelators for a variety of toxic and environmentally hazardous metals; investigating the synthesis and applications of inorganic cluster compounds; and using multiple weak interactions within small molecule receptors to target environmentally and biologically relevant substrates. The research in the group spans a diverse range of disciplines: organic synthesis of ligands and receptors; inorganic chemistry of supramolecular coordination complexes and inorganic clusters; computer modeling and ligand design; analytical chemistry of metal ion extraction; solution thermodynamics and physical organic chemistry of host-guest and metal-ligand complexes; and materials science of supramolecular assemblies and thin film semiconductors. The characterization of these nanoscale molecules requires investigation by X-ray crystallography, calorimetry, multidimensional NMR techniques, and other spectroscopic methods.
I. Supramolecular Main Group Chemistry and Specific Metal Chelation
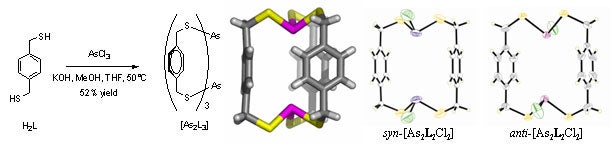
We are elaborating a design strategy for forming coordination capsules comprised of toxic metal ions or main group elements (such as arsenic, antimony, or mercury). We have recently shown that ligand H2L binds arsenic(III) within a very stable As2L3 cage. Surprisingly, two As2L2Cl2 macrocycles were also isolated as intermediates in the reaction. Applications in metal remediation, both environmental and medical, are envisioned by designing the ligands to target a specific toxic or hazardous metal ion. Furthermore, incorporating these nanoscale coordination capsules into extended solid-state structures leads to applications in materials science, and the novel cavity environments in these capsules will lead to unusual host-guest properties.
II. Inorganic Nanoclusters
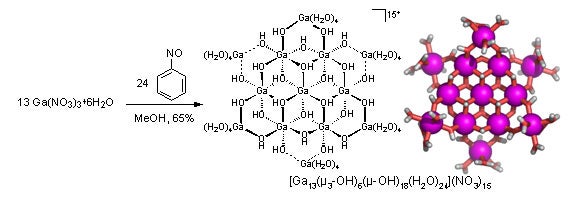
Developing predictive design strategies to prepare inorganic cluster compounds has attracted much research interest, due in part to the potential applications of these novel materials. We have developed an unusual new synthetic strategy for preparing discrete inorganic clusters, and we have used this strategy to prepare the first crystalline example of an inorganic tridecameric Ga hydroxide cluster (Ga13, see figure). A slightly altered facile synthetic methodology also yields the analogous tridecameric aluminum complex and a series of mixed Ga/In tridecameric clusters in preparative scales within one to two weeks. We are currently exploring the use of these inorganic aggregates as synthons for the generation of a wider range of particles and assemblies via exchange of the peripheral water ligands with appropriate organic ligands. In a collaboration with Prof. Douglas A. Keszler at Oregon State University, we are exploring the use of these clusters as solution precursors to prepare thin film transistor devices (Angew. Chem. Int. Ed. 2008, 9484-9486).
III. Interactions between Anions and Aromatic Rings
A recent area of study in the lab seeks to understand the how anions interact with electron-deficient aromatic rings. The related “cation-π” interaction is well-documented, but only recently has the more counter-intuitive interaction between anions and aromatic rings become appreciated. We have shown in a model system that the presence of an electron-deficient pentafluorophenyl ring enhances halide binding in solution. We have also highlighted in the solid state and in silico our observations that anions can form as many as four different attractive interactions with electron-deficient aromatic rings (J. Am. Chem. Soc. 2007, 48-58). Recently, we have used this knowledge to develop a design strategy for synthesizing tripodal anion receptors that utilize only electron-deficient aromatic rings to bind anions in solution. We seek to understand this interaction in greater detail by improving receptor design and developing new electron-deficient arenes to bind anions. Will this emerging interaction motif provide new anion binding selectivities in solution? Can new sensors and remediation materials be prepared that use these interactions to target anionic contaminants in the environment? We also have begun a new collaboration with Prof. Michael M. Haley’s lab at UO investigating the design and synthesis of modular receptors for anions based off of inherently fluorescent arylethynyl cores.
Publications
I. Supramolecular Main Group Chemistry and Specific Metal Chelation
Collins, M. S.; Carnes, M. E.; Nell, B. P.; Zakharov, L. N.; Johnson, D. W. “A Facile Route to Old and New Cyclophanes Via Self-Assembly and Capture” Nature Comm. 2016, 7, 11052.
Carnes, M. E.; Collins, M. S.; Johnson, D. W. “Transmetalation of Self-Assembled, Supramolecular Complexes” Chem. Soc. Rev. 2014, 43, 1825-1834
Cangelosi, V. M.; Zakharov, L. N.; Johnson, D. W. “Supramolecular Transmetallation Leads to an Unusual Self-Assembled P2L3 Cryptand“ Angew. Chem. Int. Ed. 2010, 49, 1248-1251.
Pitt, M. A.; Johnson, D. W. “Main Group Supramolecular Chemistry” Chem. Soc. Rev. 2007, 36, 1441-1453.
Vickaryous, W. J.; Herges, R.; Johnson, D. W. “Arsenic-π Interactions Stabilize a Self-Assembled As2L3 Supramolecular Complex“ Angew. Chem. Int. Ed. 2004, 43, 5831-5833.
II. Inorganic Nanoclusters
Fulton, B. L.; Perkins, C. K.; Mansergh, R. H.; Jenkins, M. A.; Gouliouk, V.; Jackson, Jr., M. N.; Ramos, J. C.; Rogovoy, N. M.;† Gutierrez-Higgins, M. T.;† Boettcher, S. W.; Conley, J. F.; Keszler, D. A.; Hutchison, J. E.; Johnson, D. W. “Minerals to Materials: Bulk Synthesis of Aqueous Al Clusters and Their use as Precursors for Metal Oxide Thin Films” Chem. Mater. 2017, 29, 7760-7765.
Perkins, C. K.; Eitrheim, E. S.; Fulton, B. L.; Fullmer, L. B.; Colla, C. A.; Park, D.-H.; Oliveri, A. F.; Hutchison, J. E.; Nyman, M.; Casey, W. H.; Forbes, T. Z.; Johnson, D. W.; Keszler, D. A. “Synthesis of an Aluminum Hydroxide Octamer through a Simple Dissolution Method” Angew. Chem. Int. Ed. 2017, 56, 10161-10164.
Oliveri, A. F.; Carnes, M. E.; Baseman, M. M.;† Richman, E. K.; Hutchison, J. E.; Johnson, D. W. “Single Nanoscale Cluster Species Revealed by 1H NMR Diffusion-Ordered Spectroscopy and Small-Angle X-ray Scattering” Angew. Chem. Int. Ed. 2012, 51, 10992-10996.
Mensinger, Z. L.; Gatlin, J. T.; Meyers, S. T.; Zakharov, L. N.; Keszler, D. A.; Johnson, D. W. “Synthesis of Heterometallic Group 13 Nanoclusters and Inks for Oxide Thin-Film Transistors“ Angew. Chem. Int. Ed. 2008, 47, 9484-9486.
Rather, E.; Gatlin, J. T.; Nixon, P. G.; Tsukamoto, T.; Kravtsov, V.; Johnson, D. W. “A Simple Organic Reaction Mediates the Crystallization of the Inorganic Nanocluster [Ga13(µ3-OH)6(µ2-OH)18(H2O)24](NO3)< sub>15” J. Am. Chem. Soc. 2005, 127, 3242-3243. (Highlighted inEditor’s Choice in Science, 2005, 307, 1377.)
III. Interactions between Anions and Aromatic Rings
Hartle, M. D.; Hansen, R. J.; Tresca, B. W.; Prakel, S. S.;† Zakharov, L. N.; Haley, M. M.; Pluth, M. D.; Johnson, D. W. “A Synthetic Supramolecular Receptor for the Hydrosulfide Anion” Angew. Chem. 2016, 55, 11480-11484.
Tresca, B. W.; Hansen, R. J.; Chau, C. V.;† Hay, B. P.; Zakharov, L. N.; Haley, M. M.; Johnson, D. W. “Substituent Effects in CH Hydrogen Bond Interactions: Linear Free Energy Relationships and Influence of Anions” J. Am. Chem. Soc. 2015, 137, 14959-14967.
Berryman, O.B.; Sather, A. C.; Hay, B. P.; Meisner, J. S.; Johnson, D. W. “Solution Phase Measurement of Both Weak σ and C-H•••X– Hydrogen Bonding Interactions in Synthetic Anion Receptors” J. Am. Chem. Soc. 2008,130, 10895-10897.
Berryman, O. B.; Bryantsev, V. S.; Stay, D. P.; Johnson, D. W.; Hay, B. P. “Structural Criteria for the Design of Anion Receptors: The Interaction of Halides with Electron-Deficient Arenes” J. Am. Chem. Soc. 2007, 129, 48-58.
Berryman, O. B.; Hof, F.; Hynes, M. J.; Johnson, D.W. “Anion-π Interaction Augments Halide Binding in Solution” Chem. Commun. 2006, 506-508.
